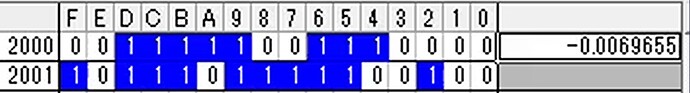
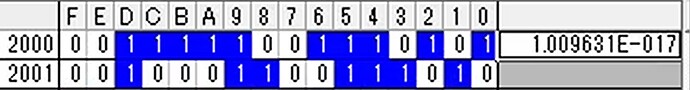
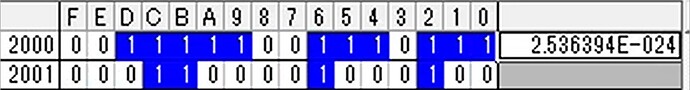
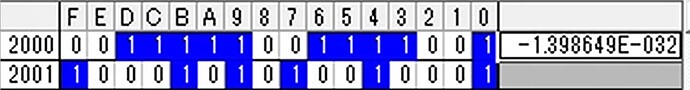
Hi, I’m tring to connect NH3 sensor.But NH3 value that I got seems not correct. After start up, output value became 00000000 (HEX), if I placed the sensor to ammonia environment (e.g. around toilet), the value became strange value (e.g. A5F84086 (HEX)) and did not stable. (Sometime the value is also not Float32 format.) I could get humidity and temperature value correctly, so I think my reading setting of address of NH3 value (0x2000) is also correct. In this case, is sensor something wrong?
Upload the received data from NH3 sensor every second. I think the last (5.jpg) is strange data especially.
I’m sorry, This issue was a PC side issue and has been resolved.
I am also having the same issue that the value from register is 00 00 00 00 and for make sure the sensor is working sensor is exposed to Ammonia Gas and it reached its maximum of 100 ppm and after some time it get back to 0 ppm as before and we have another ammonia sensing device for comparing and that device gives the result as 0.5 to 2 ppm in normal condition of a testing area.
And more importantly we are powering the Sensor only when we need data and once the reading is completed then sensor power is in off State so that the sensor is not in continues ON State(as we are looking for remote application battery operated mode so we are not able to keep the Sensor ON for all the time).
And the sensor tolerance is about 5 - 10 ppm as per Datasheet weather this may cause any difference as our comparing device gives only 0.5 to 2 ppm in testing field.
Kindly advice me on this…
Hi,
Could you please take some photos to show the environment where the sensor is placed? Additionally, please send me some screenshots of the data.
How do you receive this data? Do you connect a data logger to collect the data or just use the RS485 command?
I am using RS-485 only. Datalogger is not used in our application.
We just read the data and sent the data to cloud there is no need of logging here, so we only use Sensor with RS-485 module.
I attached the photos of the testing environment and Data’s we got in serial port for your reference.
I can’t able to put photos here. It shows the warning message as “Sorry,you can’t embed media files here”
try using the datalogger and see if it confirms your numbers
Log the raw data from the sensor over a period of time to analyze any patterns or irregularities.
Thanks for your responses,
I changed the testing environment to another restroom with low ventilation, now sensor returns ammonia value.
But as the range of the Sensor is 0-100 ppm I am having the doubt that the mild range of Ammonia (0-2 ppm) was not measured with good Accuracy (Now Sensor gives 0-1.3 ppm in Testing Environment).
Can you guys suggest me some technique verify the value measured is correct! or is there any other sensor available to measure mild level of Ammonia presence.
Hi there,
Ammonia is lighter than air and collects at the ceiling (or the highest point of the room). Therefore the gas detector/sensor should be mounted on or at the ceiling .
here is a good thread about testing different sensor types.
and some basics it states, If you were to mount the detector at breathing height (1.2 - 1.8 m / 4 - 6 ft from the floor), the concentration of ammonia would start at the ceiling and move down towards the breathing height as more and more leaked and collected. By the time the ammonia level was at breathing height, the room would be so full of ammonia, not only would it be a breathing hazard, but also an explosion/fire hazard, not to mention the high cost of losing all that ammonia. The goal is to be alerted of an ammonia leak as soon as possible so safety measures can be taken to stop and repair it.
NOTE: In the presence of moisture (such as high relative humidity), ammonia gas forms vapour that is heavier than air and may spread along areas with poor air flow where people may be exposed.
HTH
GL ![]() PJ
PJ
![]()
you can dilute some to a 50/50 solution and after a sample remove it and apply compressed air for a few minutes and retest it should change close to Zero and repeat the test a few times to get some sample levels. You won’t get Zero, unless it’s in a vacuum or in nitrogen zero gas stream.
Thank you for your reply @PJ_Glasso
wow i didnt even think of the lighter than air part! i think he is using pee ammonia in the restroom… but yes ammonia is used in industrial refrigeration
Hi there,
Well in the chip Fabs in OR. TSMC and Fujitsu they use a lot of it along with some shit that is pyrophoric if it hits oxygen (down in TX afew years ago) a couple techs Expired ![]() from it being in the tunnel between buildings after a leak. Gases are no joke when not monitored properly, I built a Hydrogen sensor that the company still uses today as our own canary in the UHP gas pad and cabinets…
from it being in the tunnel between buildings after a leak. Gases are no joke when not monitored properly, I built a Hydrogen sensor that the company still uses today as our own canary in the UHP gas pad and cabinets…
some of that stuff btt you smell it your DOA! ![]()
HTH
GL ![]() PJ
PJ
![]()
probably why I got those Sinus polyps I just had surgery for. Feeling a buc 100 now ![]()
We got meat packing plants with tank farm sized ammonia tanks and wind socks to show the direction if a leak is reported… also i worked in the sewer industry and sewer gasses have sulpheric acid and confined entry spaces and all that stuff… also chlorine gas… a train car derailed and killed a whole town with it… What Da?
there is also a difference between anhydrous ammonia and regular ammonia and how it works
One is with water and one without, The later is illegal btw (anhydrous ammonia)
they both work the same and do the same it’s how big of a reaction in a gas or chamber you want to achieve. The chip Fabs use it for a purifier process.
is all I know about it.
Don’t smell it… ![]()
![]()
![]()